News

Americans Believe Government Should Require Copay Assistance Be Applied to Out-of-pocket Costs
Advocacy & Legislation, Industry News & Research
The election is right around the corner and there’s a lot of information to absorb. That is why the National Hemophilia Foundation…

Patient Advocacy Organizations Release Blueprint to Protect Access to Care
Advocacy & Legislation, Industry News & Research
A coalition of 33 organizations, including the National Hemophilia Foundation, representing millions of people with pre-existing conditions launched an unprecedented effort today…

GET WIRED WITH HFA
Industry News & Research, Living with a Bleeding Disorder, Women with Bleeding Disorders
Feeling disconnected from knowledge about women’s health and research? Get WIRED with the Hemophilia Federation of America (HFA) is seeking women with bleeding…

Public Health Symposia Now Available on the NHF Website!
Industry News & Research
This summer’s Virtual Bleeding Disorders Conference (BDC) featured a pair of excellent public health symposiums, which were developed through an inspired collaboration…

First Patient Dosed in Phase 3 Gene Therapy Trial
Industry News & Research
The purpose of the study is to evaluate the efficacy and safety of this investigational gene therapy in patients with moderately severe…

Unanswered Questions Remain in Gene Therapy Trial
Industry News & Research
This commentary was authored by six individuals with severe hemophilia, all of whom have worked to achieve better health outcomes for patients…

What’s Next for the ACA?
Advocacy & Legislation, Industry News & Research
The recent news about the passing of United State Supreme Court Justice Ruth Bader Ginsburg and nomination of Judge Amy Coney Barrett…

CSL Behring States Stimate Will Not Be Resupplied until 2022
Industry News & Research
NHF and HFA have been provided the following statement from CSL Behring. The statement says, in part: “Ferring Pharmaceuticals Inc. initiated a voluntary,…

Milwaukee County COVID-19 Update
COVID-19, Industry News & Research
The Milwaukee Unified Emergency Operations Center continues to put out up-to-date information for the citizens of Milwaukee County. This information is also…

NHF Wants Your Input as They Chart a Course for the Future. Take Their Survey Today!
Industry News & Research
The National Hemophilia Foundation (NHF) is working with the bleeding disorders community to envision our future for the next decade, and they…

NHF Friday Webinar – Gene Therapy: What’s New and What’s Next? October 2, 3 pm.
Industry News & Research
Gene therapy for hemophilia remains a very active area of research, although it may take longer than expected. To discuss this, join…

Emergency Preparedness
Industry News & Research
The NHF is saddened by the loss of life and destruction of communities due to catastrophic fires in the west and a…

Unanswered Questions Remain in Gene Therapy
Gene Therapy, Industry News & Research, Living with a Bleeding Disorder
A recent commentary published in the journal Haemophilia, posits that while clinical trials for hemophilia gene therapy approach conclusion, there remain significant unanswered…

Sanofi Publishes Updates to BIVV001 Trial
Industry News & Research
Sanofi recently announced results from their phase 1/2a EXTEN-A trial for their investigational therapy BIVV001, which were published in the New England Journal…

Hemophilia B Therapy to be Discontinued
Industry News & Research
CSL Behring recently announced that they are discontinuing Mononine®, the company’s human plasma-derived factor therapy indicated for the prevention and control of…

NHF’s Board of Directors Meeting
Industry News & Research
The National Hemophilia Foundation will be holding our quarterly Board of Directors meeting on Saturday, October 10th at 9:00 AM EDT. The Board…

Genentech Provides Updates on Hemlibra Product Particles
Industry News & Research
In the fall of last year Genentech reported that during a routine examination of batches of Hemlibra®, they found translucent particles composed…

NHF, HFA and Hemophilia Alliance Continue Push for Information on Stimate Recall
Industry News & Research
The National Hemophilia Foundation (NHF) and Hemophilia Federation of America (HFA) continue to follow up on issues arising from the recent recall of Stimate (desmopressin)…

New MASAC Recommendations Focus on Offsite Hemostasis Screening and Treatment
Industry News & Research
The National Hemophilia Foundation’s (NHF) Medical and Scientific Advisory Council (MASAC) issued two new documents, which were adopted by NHF’s Board of…

Mental Health During Coronavirus
COVID-19, Industry News & Research
by: Debbie de la Riva The collective pursuit to control the spread of coronavirus has resulted in an enormous challenge for the…

NHF’s 2020 Bleeding Disorders Conference Available On Demand Through September 30!
Industry News & Research
NHF’s Bleeding Disorders Conference was fully virtual this year and included more than 50 sessions. Those sessions are all available on-demand until…
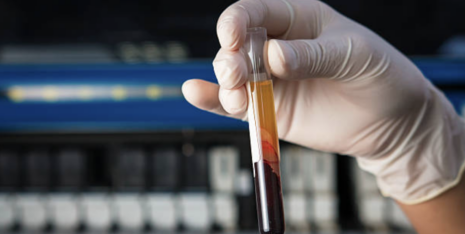
BioMarin’s New Clinical Study to Focus on Antibody Development to AAVs
Industry News & Research
Last month, BioMarin learned that approval for their investigational hemophilia A gene therapy was not forthcoming but would in fact be delayed…

Mylan Initiates Voluntary Nationwide Recall of Four Lots of Tranexamic Acid Injection, USP Due to Carton Label Mix-Up
Industry News & Research
On September 1, 2020, NHF and HFA learned that Mylan N.V. is conducting a voluntary nationwide recall of four lots of Tranexamic Acid Injection,…

NHF Receives Five-Year CDC Grant for Education Programs
Industry News & Research
The National Hemophilia Foundation (NHF) has received a five-year cooperative agreement from the Centers from Disease Control and Prevention (CDC). The collaboration,…