UPDATE: Takeda to Voluntarily Replace Certain BAXJECT® II Reconstitution Devices
Takeda, in agreement with the U.S. Food and Drug Administration (FDA), has decided to voluntarily replace BAXJECT® II reconstitution devices produced by Baxter between October 2021 and January 2022 co-packaged for use in conjunction with RECOMBINATE™ [Antihemophilic Factor (Recombinant)] and RIXUBIS® [Coagulation [Factor IX (Recombinant)].
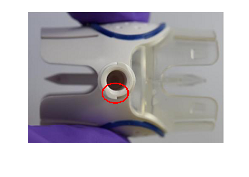
Takeda has received reports of white particles identified near the luer port of the BAXJECT II device (see images below). All reported incidents to date were observed prior to administration, either when the luer port cap was removed as part of the preparation process or in the syringe after the drug was reconstituted. It is important to note there is no quality issue with either RECOMBINATE and/or RIXUBIS drug product, and therefore the products are safe for use. No particles have been identified in the active product prior to reconstitution. There were no reported adverse events attributable to the presence of particles in the BAXJECT II device in our Takeda Global Safety databases leading to the voluntary replacement of the BAXJECT II reconstitution device. The safety and efficacy profiles of RECOMBINATE and RIXUBIS remain consistent with the product Prescribing Information.
Takeda will provide replacement BAXJECT II reconstitution devices to pharmacy providers that have received impacted lots for distribution to patients and reconstitution of product per the Instructions for Use. Please see the following information regarding steps for contacting your pharmacy provider to request replacement BAXJECT II device(s).
Replacement of Impacted Devices
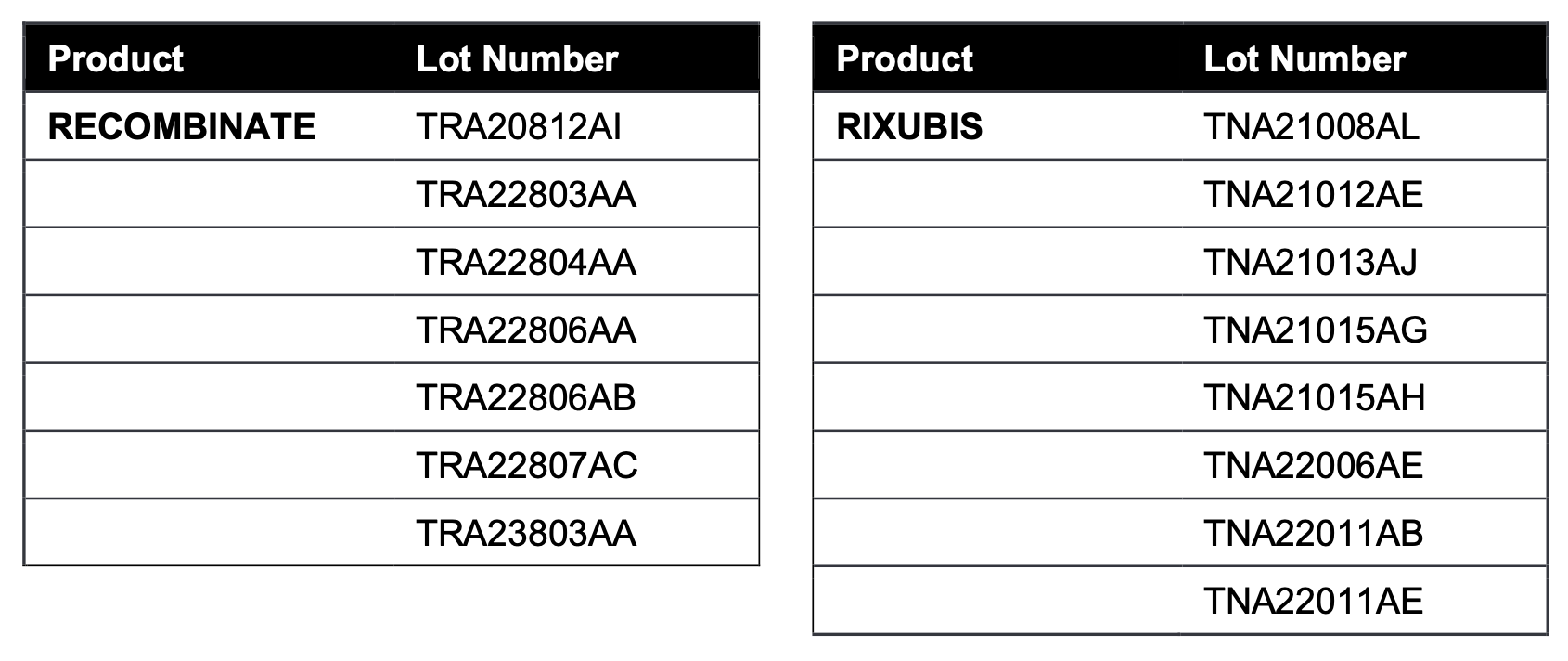
The impacted lots of BAXJECT II DEVICES co-packaged with RECOMBINATE and RIXUBIS are listed below:
Please examine any RECOMBINATE and/or RIXUBIS product in your possession to identify any impacted lots. You can locate the lot number on the outside of the product packaging (see image below).
• If you have impacted RECOMBINATE and/or RIXUBIS product lots in your possession, please contact your pharmacy provider to request replacement BAXJECT II device(s). The pharmacy provider will ship the replacement device(s) directly to you.
• Upon receipt of the BAXJECT II replacement device, you should carefully follow the Instructions for Use for preparation and administration of the RECOMBINATE and/or RIXUBIS product.
• In the Instructions for Use, when prompted to open the package of the BAXJECT II device contained within the kit, the patient should discard the original BAXJECT II device and use the new replacement BAXJECT II device provided.
• If you have further questions, please contact your pharmacy provider or prescribing healthcare provider directly.
Reporting Adverse Events
Healthcare providers and patients are encouraged to report adverse reactions and/or quality problems related to the BAXJECT II reconstitution device, RECOMBINATE and/or RIXUBIS to Takeda at 1-877-TAKEDA-7 (1-877-825-3327). You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please know that patient safety and supply continuity are our top priorities, and we are working urgently to provide replacement BAXJECT II devices to customers.
UPDATE as of 08/24/2023
The Medical and Scientific Advisory Council of the National Bleeding Disorders Foundation issued the following medical advisory on an issue with certain BAXJECT® II reconstitution devices:
The lot numbers involved in this advisory:
Takeda has brought to our attention an issue with some BAXJECT® II reconstitution devices produced between October 2021 and January 2022 for use in conjunction with RECOMBINATE [Antihemophilic Factor (Recombinant)] and RIXUBIS® [Coagulation Factor IX (Recombinant)] in the U.S. First reported 16 August 2023 here.
Takeda notified the U.S. Food and Drug Administration (FDA) of reports of plastic particles originating near the luer port of the BAXJECT II device. All reported complaints to date were observed prior to administration, either when the luer port cap was removed as part of the preparation process or in the syringe after the drug was reconstituted. There have been no reported adverse events attributable to the BAXJECT II device to date. These particles reportedly are too large to transfer from the contaminated medication through the needle, tubing, and to the patient.
BAXJECT II Reconstitution Device

Luer Port on the Device
Takeda reports that ADVATE, ADYNOVATE and other Takeda products are not affected since the BAXJECT II device is only packaged for use with RIXUBIS and RECOMBINATE in the United States. Takeda is working with the FDA to obtain appropriate guidance for next steps, and specific lot numbers of affected factor batches are not available currently. In the interim, MASAC recommends patients using RECOMBINATE and RIXUBIS review and visually inspect their medication supply carefully before injection, both in the bottle, and in the syringe. The reconstituted solution should look colorless to faint yellow, and free from foreign particles. In the event particles are identified in the reconstituted product, please do not administer the product. MASAC advises patients using RECOMBINATE and RIXUBIS® who may have been affected to contact their treatment center if applicable. Alternatively, patients could consider mixing their product using an alternate method, such as with the use of a filter needle attached to the end of syringe during product mixing. For instructions on how to do this, and how to obtain supplies, contact your treatment center or pharmacy. Patients can also contact their treatment center or pharmacy to re-order new factor product that is not packaged with the BAXJECT II device manufactured in the aforementioned time period. MASAC will continue to work disseminate additional information as it is available.
Healthcare providers and patients are encouraged to report adverse reactions and/or quality problems related to the BAXJECT II reconstitution device, RECOMBINATE and/or RIXUBIS to Takeda at 1-877-TAKEDA-7 (1-877- 825-3327). You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Source: Hemophilia Federation of America (HFA), September 2023